We value your privacy
We use cookies to enhance your browsing experience, serve personalized ads or content, and analyze our traffic. By clicking "Accept All", you consent to our use of cookies.
We use cookies to help you navigate efficiently and perform certain functions. You will find detailed information about all cookies under each consent category below.
The cookies that are categorized as "Necessary" are stored on your browser as they are essential for enabling the basic functionalities of the site. ...
Necessary cookies are required to enable the basic features of this site, such as providing secure log-in or adjusting your consent preferences. These cookies do not store any personally identifiable data.
No cookies to display.
Functional cookies help perform certain functionalities like sharing the content of the website on social media platforms, collecting feedback, and other third-party features.
No cookies to display.
Analytical cookies are used to understand how visitors interact with the website. These cookies help provide information on metrics such as the number of visitors, bounce rate, traffic source, etc.
No cookies to display.
Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors.
No cookies to display.
Advertisement cookies are used to provide visitors with customized advertisements based on the pages you visited previously and to analyze the effectiveness of the ad campaigns.
No cookies to display.
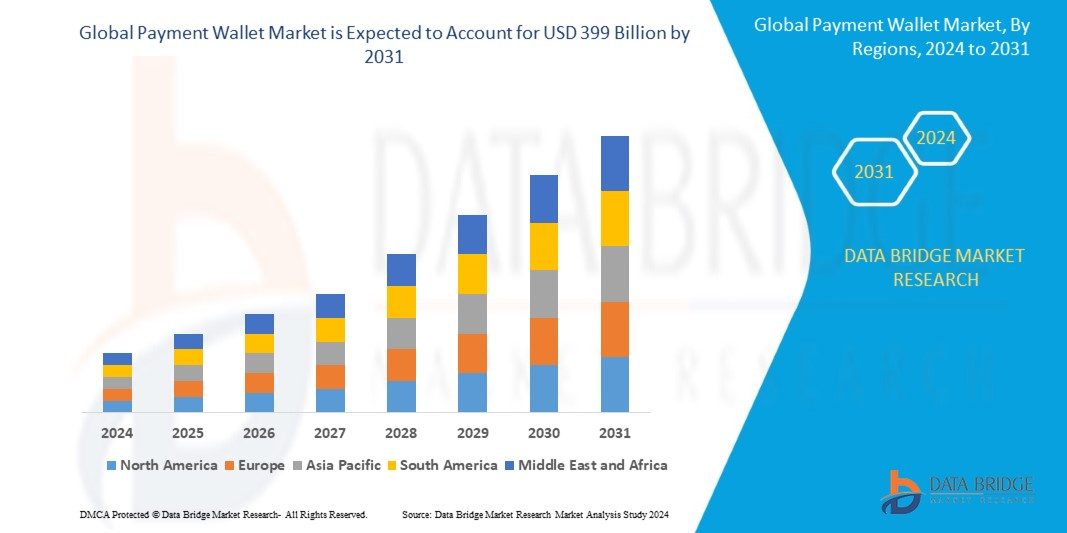
Data Bridge Market Research analyzes that the global payment wallet market size is valued at USD 100.2 billion in 2023 and is predicted to reach USD 399 billion by 2031 by the year 2031 at a 16.8% CAGR during the forecast period for 2024-2031.

Are you looking for great furniture to make your home look amazing? Do you want something special that no one else has? Then you are in the right place! If

Global Polyethylene Market Poised to Reach USD 165.88 Billion by 2032, Driven by Demand Across Packaging, Automotive, and Construction Sectors The global Polyethylene Market Size is projected to expand from USD 126.95 billion
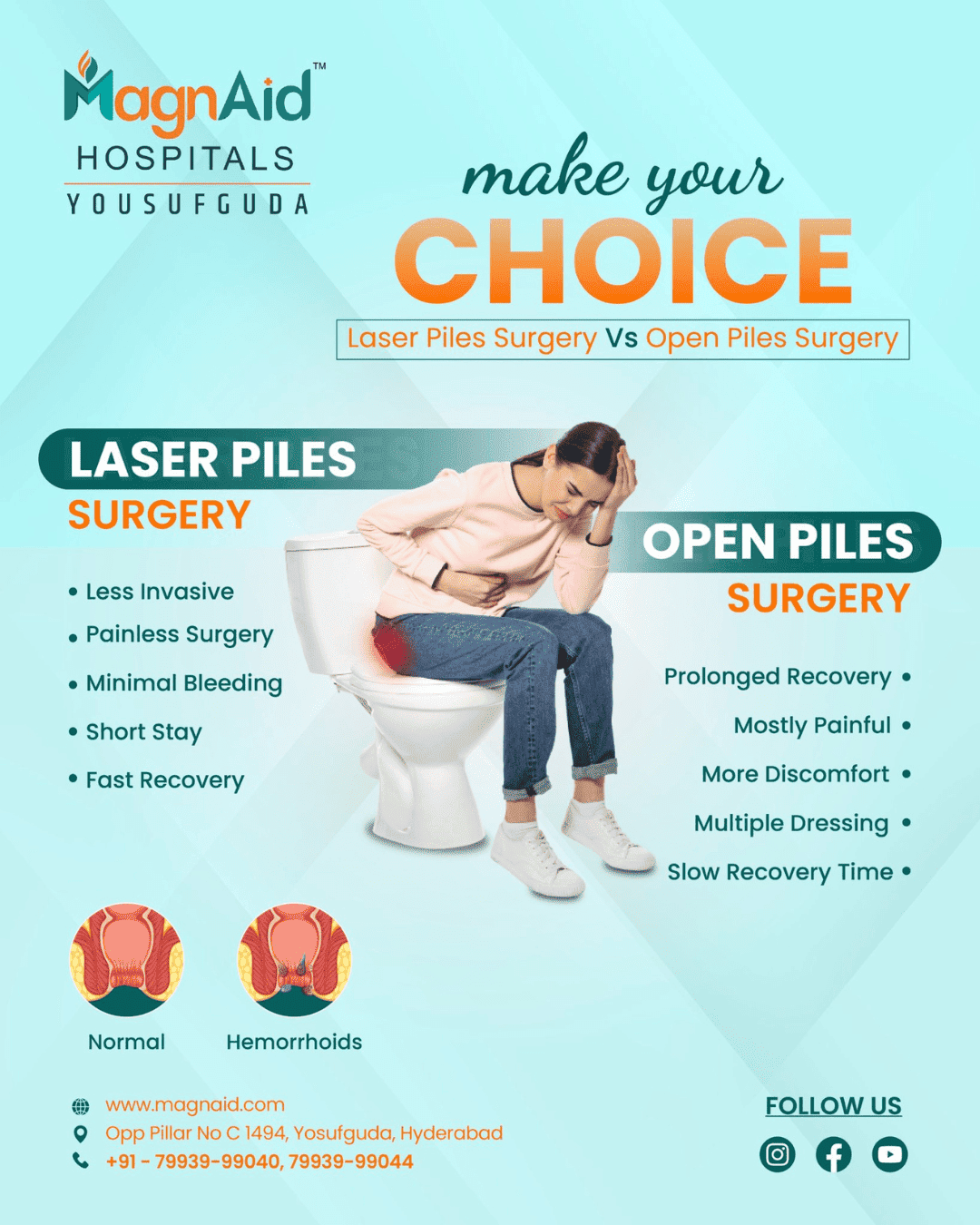
General surgery is a specialised branch of medical science which deals with treating different conditions within the body through surgical methods.

Confused about home loan interest rates? This easy guide explains what they are, how they work, and how to choose the best rate for your budget. Introduction Buying a home

If you love exploring new places or travel frequently for work, a good travel-focused credit card can help you save more and enjoy better travel experiences. From lounge access to

Understanding the Complex Landscape of Dubai Real Estate Dubai’s real estate market is one of the most dynamic and fast-growing in the world. From luxury villas to commercial skyscrapers, the

Navigating Deportations from UK, How Immigration Solicitors4me Supports Clients Facing Removal

Experience top seafood, dock and dine, and outdoor dining at Breakwater—one of the best restaurants in Stonington Borough, CT.

With the growth of competition, companies are looking to smarter, cloud-based solutions to optimize their operations and improve customer interaction. One such powerful solution is SAP Cloud for Customer (SAP C4C)— a cloud-based CRM system that helps integrate services, sales, and marketing for maximum productivity. Enroll now in ERPVITS for the SAP C4C course and take the first step towards becoming an expert in SAP cloud CRM.

Dive into the amazing realm of Comme des Garcons. Discover fearless fashion choices that will energize your look and make you stand out today

Some of the most famous collage artists, spanning across different eras and styles, have made significant contributions to the world of art.

Blood cancer is a serious condition that affects the body's ability to produce and regulate blood cells. The disease typically originates in the bone marrow or lymphatic system, leading to

In this guide, we’ll take a closer look at what to expect from skip hire in Mitcham and Sanderstead, why it’s essential during home renovations, and how local skip providers can make the process seamless.

In today’s hyper-connected world, establishing a strong digital presence is no longer optional — it’s essential. Whether you’re a local startup or a well-established business in Lucknow, the digital landscape offers immense opportunities for growth. With consumers turning to the internet for everything from product research to purchasing decisions, businesses that invest in digital marketing stand to gain a competitive edge.

Do you love cooking with fresh and tasty spices? Do you want to buy large amounts of spices at once for your business or home? Then you need a trusted

Agile methodology has revolutionized the way custom software development projects are approached. It focuses on flexibility, collaboration, and iterative development, ensuring that businesses get the most value from their software development efforts. In this article, we’ll dive into how Agile works within custom software development and why it’s a smart approach for modern businesses.

Learn how PedroVazPaulo Real Estate Investment helps families build generational wealth through smart property strategies and long-term planning.

Understanding how to style your watch properly shows attention to detail and confidence in your fashion sense. Here’s how you can pair belt watches with various outfits without overthinking it.

Mayank Singhvi’s career is a bright illustration of what can be accomplished with vision, strategy, and commitment for companies and investors seeking inspiration and leadership in the always-changing environment of private equity.




















Ranks rocket connects website owners with bloggers for free guest posting! Increase brand awareness and backlinks with strategic placements. But remember, quality content is key.