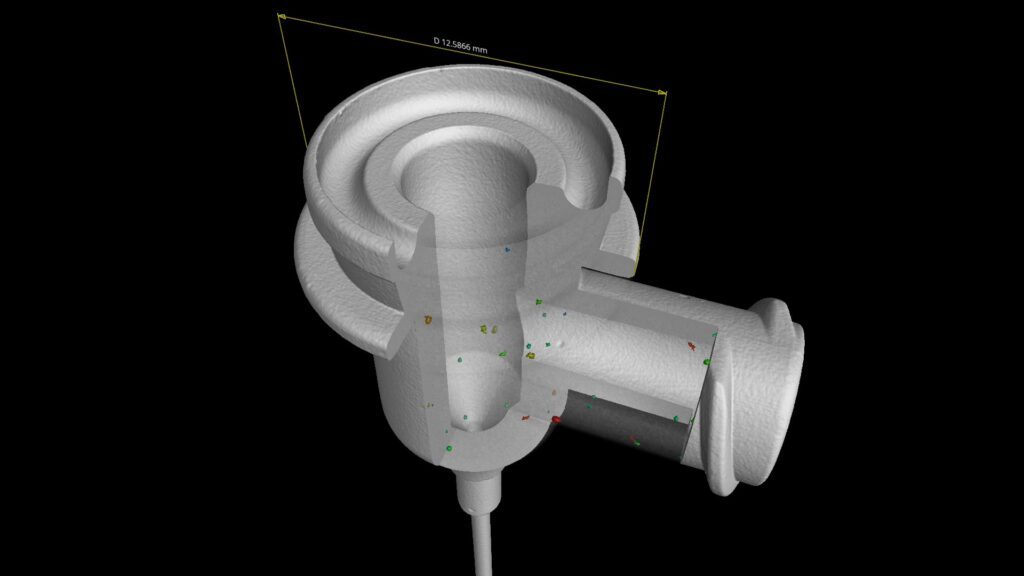
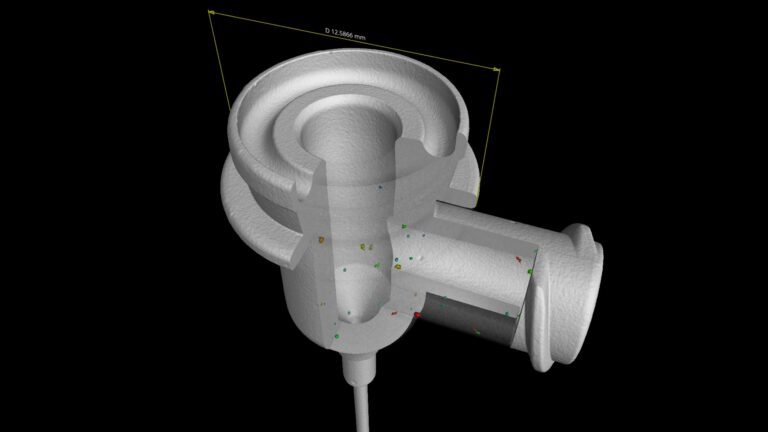
Introduction
In the medical device industry, patient safety is not optional—it is the foundation of every design, test, and regulatory requirement. One of the most critical components in many healthcare applications is the small but essential connector used to deliver fluids and medications. Luer connectors are widely used in syringes, catheters, infusion sets, and other medical devices. However, incorrect connections between incompatible devices have historically caused serious patient harm.
To address these risks, the ISO 80369 series was developed to standardize small-bore connectors and reduce the possibility of misconnections. Luer ISO 80369 testing plays a crucial role in verifying that connectors meet strict dimensional, mechanical, and performance requirements. Manufacturers must conduct this testing to ensure compliance, product reliability, and global market acceptance.
This article explores what Luer ISO 80369 testing involves, why it matters, and how manufacturers can ensure their devices meet the necessary standards.
What is ISO 80369?
ISO 80369 is an international standard that specifies design and testing requirements for small-bore connectors used in healthcare applications. It was developed to minimize the risk of accidental connections between unrelated delivery systems, such as enteral, respiratory, neuraxial, and intravenous systems.
The ISO 80369 series includes multiple parts, each focusing on specific connector types. For Luer connectors, the relevant standard is ISO 80369-7, which defines requirements for intravascular or hypodermic applications.
Luer connectors are commonly used in:
-
Syringes
-
Intravenous (IV) sets
-
Catheters
-
Infusion lines
-
Blood collection systems
Because these connectors are widely used across medical environments, strict compliance is essential to ensure patient safety and functional compatibility.
Why Luer ISO 80369 Testing is Important
Luer connectors may appear simple, but even small dimensional variations can result in leakage, disconnection, or misconnection. Luer ISO 80369 testing ensures:
1. Prevention of Misconnections
Incorrect device connections can cause serious injury or even fatal outcomes. ISO 80369 testing confirms that Luer connectors cannot easily connect to incompatible systems.
2. Leak Prevention
Fluid leakage in intravenous systems can lead to contamination, dosing errors, and infection risks. Testing verifies the connector’s sealing integrity under pressure.
3. Mechanical Strength
Connectors must withstand tensile forces, torque stress, and repeated use without failure. Mechanical performance testing ensures structural durability.
4. Regulatory Compliance
Compliance with ISO 80369-7 is often required for CE marking, FDA submissions, and other regulatory approvals worldwide.
Without proper Luer ISO 80369 testing, manufacturers risk product recalls, regulatory rejection, and potential legal consequences.
Key Components of Luer ISO 80369 Testing
Testing under ISO 80369-7 typically includes dimensional verification and performance validation. Below are the core testing categories.
Dimensional Testing
Dimensional accuracy is critical for compatibility and safety. Testing laboratories use calibrated gauges and measurement tools to verify:
-
Taper dimensions
-
Inner and outer diameters
-
Thread specifications (for Luer lock connectors)
-
Length and engagement depth
Even minor deviations can result in improper sealing or mechanical instability.
Leakage Testing
Leakage testing evaluates the connector’s ability to maintain a seal under internal pressure. The device is subjected to specified pressure levels while monitoring for fluid or air leaks.
This ensures that Luer connectors can safely deliver fluids without failure during real-world medical procedures.
Separation Force Testing
Separation force testing measures the amount of force required to disconnect the connector. The connector must not separate under normal clinical use but should still allow intentional disconnection without excessive force.
Resistance to Overriding
Overriding occurs when one connector is forced over another improperly. ISO 80369 testing evaluates resistance to such misuse, helping prevent accidental cross-connections.
Stress Cracking and Durability
Materials used in Luer connectors must withstand repeated mechanical stress and exposure to medications or disinfectants. Testing confirms that connectors maintain performance over time.
Testing Process and Laboratory Requirements
Luer ISO 80369 testing must be conducted in accredited laboratories using standardized methods and calibrated equipment. The general process includes:
-
Sample preparation according to production specifications
-
Environmental conditioning (temperature and humidity control)
-
Dimensional verification using precision instruments
-
Mechanical and leakage performance testing
-
Documentation and reporting of results
Manufacturers should ensure testing aligns with both ISO 80369-7 and applicable regulatory requirements for their target market.
Common Challenges in Luer ISO 80369 Testing
Despite clear standards, manufacturers often face challenges such as:
-
Design tolerances that barely meet dimensional limits
-
Material compatibility issues
-
Failure during torque or pressure testing
-
Inconsistent production quality
To avoid repeated failures, early-stage design validation and prototype testing are strongly recommended before mass production.
Best Practices for Manufacturers
To ensure successful compliance with Luer ISO 80369 testing, manufacturers should:
-
Integrate ISO 80369 requirements during the design phase
-
Conduct internal pre-compliance testing
-
Use high-precision molds and quality-controlled materials
-
Partner with experienced accredited testing laboratories
-
Maintain detailed documentation for regulatory audits
Proactive quality control reduces testing delays and speeds up regulatory approval.
Conclusion
Luer ISO 80369 testing is not just a regulatory requirement—it is a critical safeguard for patient safety and device reliability. By verifying dimensional accuracy, mechanical strength, and leakage resistance, manufacturers can ensure that their Luer connectors perform safely in clinical environments.
As global healthcare regulations continue to emphasize risk reduction and standardization, compliance with ISO 80369-7 has become essential for market access and brand credibility. Manufacturers who prioritize thorough Luer ISO 80369 testing during product development are better positioned to deliver safe, compliant, and high-quality medical devices.
In a highly regulated and patient-sensitive industry, precision is not optional. Testing ensures that every connection performs exactly as intended—protecting both patients and healthcare professionals alike.